CMC 개발 전문성을 바탕으로 한 신약개발시스템
제넥신은 혁신신약개발을 위한
CMC 개발 전문성을 보유하고 있습니다.
시설 및 CMC 전문가 그룹
- Facility
- · Small scale(3L, 15L)
- · Large Scale(50L, 200L)
- · Analysis : LC-MS & MS/MS, HPLC-UV/FLD/ELSD, CE & Bioanalysis system
- CMC Experts
- · Ph.D., Masters, experts
- · Scale up(up to 200L)
- · Formulation study
- · Method Development & Qualification
- · Tech Transfer(USP, DSP, Analysis)
- · Quality Management System
- · CTD documentation(IND, NDA)
- CMO & CRO Management
- · Cell bank
- · DS/DP Manufacturing
- · UPB, VC study for IND filing
- · Toxicity study
- · DS/DP Release, Stability
- Process Development Up to Clinical
- · Ph2 project 3(KR, US, EU) : GX-I7, GX-H9, GX188E
- · Ph3 project 1(KR, Indonesia) : GX-E4
임상을 위해 지원되는 생물학적 제제 플랫폼
제넥신은 한국, 미국, 유럽에서 임상시험 공정 개발 경험을 보유하고 있습니다.
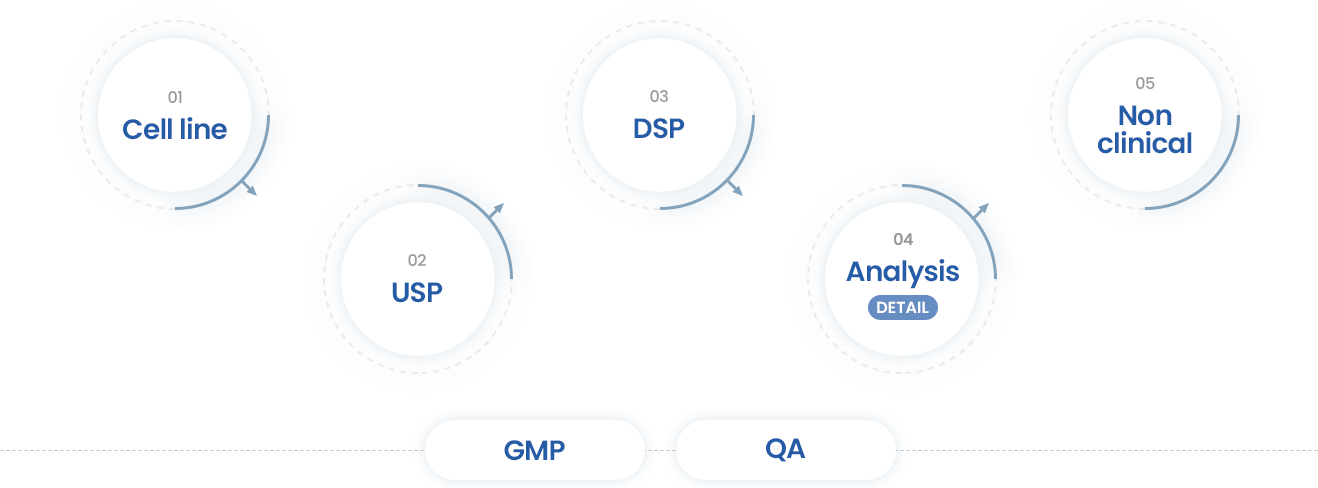
- 01Cell line
- · Support the Cell line Dev.
- · Using CMO/CRO
- 02USP
- · Cell culture Dev.
- · Scale up (~200L)
- · Support the UPB using CRO
- · IND/NDA filling
- 03DSP
- · Purification Dev.
- · Scale up(~200L)
- · Support the VC using CRO
- · IND/NDA filling
- 04Analysis
- · Method dev. Of HPLC, CE & Bio assay dev.
- · Formulation study
- · Experimental study
- · Support the CRO/CMO
- · IND/NDA filling
- · Full Characterization
(Protein, Glycan, Physicochemical Properties, Impurity)
- 05Non clinical
- · Analysis
- · Support the toxicity study using CRO
- · Method dev. Of Bioanalysis, ADA, and DFA for protein drugs based on the GLP system
- GMP
- · Support the GMP
- · Tech Transfer to CMO
- · Review the Batch Record of DS/DP Mfg
- · DS/DP Release, Stability Report Review
- QA
- · Multiple CMO management
- · Quality Management System